Hydrogen the most abundant element in the Universe, if you could find an efficient way to
extract it out of water, economies all across the world would benefit.
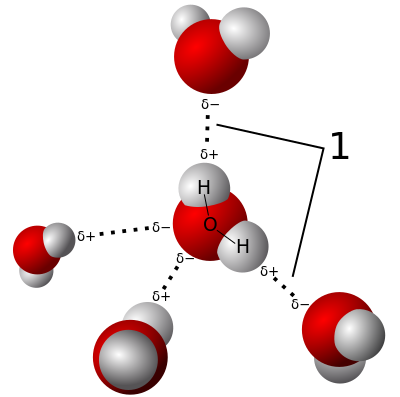 | |
| 1 =Hydrogen water bonds |
Many people in many countries are experimenting as you read this page.
They are not exclusively main stream scientists, some are ordinary, science
interested people, but not with qualifications or degrees.
interested people, but not with qualifications or degrees.
Amateurs have no preconceptions, they are not hindered by fixed ideals, so
maybe they will stumble upon ( as do many main stream scientists ),
new outside the box ideas.
new outside the box ideas.
several methods are used to break the Hydrogen/Oxygen bonds.
Electrolysis is a good method, but...... it uses a lot of energy to break the covalent
bonds of Hydrogen H2 and Oxygen O.
 |
| Animation by ;Brian0918/ Wikipedia |
Even our DNA have bonds that use Hydrogen
A typical setup for extracting Hydrogen and Oxygen, the operating voltage should be around 1.2 volts DC. If you recombine the Oxygen with the Hydrogen you will have
a VERY volatile mixture, that is more explosive than Hydrogen alone. It can be used
in a car ( I don't endorse use in a automobile) either directly into cylinders or to fuel a fuel cell. This combined mixture is called "browns gas", in this form it should NEVER be
stored under pressure, as autoignition occurs at about 570 °C (1065 °F).
I do have a plan in the future to assist the Fuel consumption in my own car ( not on the Road )
but it can not be used in cars with computer controlled engines. Or EMS (Engine management systems). My car is very old and does not even have a exhaust catalyzer, so
It will not effect the set air/petrol mixture entering the engine.
My design is based on an multi stainless steel / Electrolytic cell, with a PWM (pulse width modulator) to drive it. See My assembled unit below. But not yet wired or tested.
I will post any test results I gain in a future post.
In the news recently is a new method of producing Hydrogen, without using Sun light/electricity or chemical additives. It would make it
more efficient (Maybe?)than current methods. It uses spherical silicon particles
in water to produce Hydrogen, the silicon is only 10 nanometers in diameter!, a human hair is approx 90,000 nanometers wide so
10 nanometers must be smoke particle size, so its going to take a fare amount of
energy to make silicon spherical's that small, but if you could produce enough
Hydrogen to self loop the process of making the spherical silicon particles, with
energy in reserve then it would be a top technology. Original story from; http://www.sciencedaily.com/releases/2013/01/130122143224.htm
So get inventing solve this problem, solve world energy needs.
A typical setup for extracting Hydrogen and Oxygen, the operating voltage should be around 1.2 volts DC. If you recombine the Oxygen with the Hydrogen you will have
a VERY volatile mixture, that is more explosive than Hydrogen alone. It can be used
in a car ( I don't endorse use in a automobile) either directly into cylinders or to fuel a fuel cell. This combined mixture is called "browns gas", in this form it should NEVER be
stored under pressure, as autoignition occurs at about 570 °C (1065 °F).
 | |
| B.Arrowsuch 2013 |
I do have a plan in the future to assist the Fuel consumption in my own car ( not on the Road )
but it can not be used in cars with computer controlled engines. Or EMS (Engine management systems). My car is very old and does not even have a exhaust catalyzer, so
It will not effect the set air/petrol mixture entering the engine.
My design is based on an multi stainless steel / Electrolytic cell, with a PWM (pulse width modulator) to drive it. See My assembled unit below. But not yet wired or tested.
I will post any test results I gain in a future post.
In the news recently is a new method of producing Hydrogen, without using Sun light/electricity or chemical additives. It would make it
more efficient (Maybe?)than current methods. It uses spherical silicon particles
in water to produce Hydrogen, the silicon is only 10 nanometers in diameter!, a human hair is approx 90,000 nanometers wide so
10 nanometers must be smoke particle size, so its going to take a fare amount of
energy to make silicon spherical's that small, but if you could produce enough
Hydrogen to self loop the process of making the spherical silicon particles, with
energy in reserve then it would be a top technology. Original story from; http://www.sciencedaily.com/releases/2013/01/130122143224.htm
So get inventing solve this problem, solve world energy needs.


No comments:
Post a Comment